For years, they had been losing their central vision—what allows people to see letters, faces, and details clearly. The light-receiving cells in their eyes had been deteriorating, gradually blurring their sight. But after receiving an experimental eye implant as part ...
As mpox continues to spread in Central Africa, a promising antiviral drug to treat the infection has failed to improve patients’ symptoms in a trial in the Democratic Republic of the Congo, the epicenter of the outbreak. In the trial, ...
Amid a summer surge of Covid-19 infections, the US Food and Drug Administration just approved updated mRNA vaccines that more closely target the currently circulating variants of the coronavirus. The updated vaccines, from Moderna and Pfizer/BioNTech, target a variant of ...
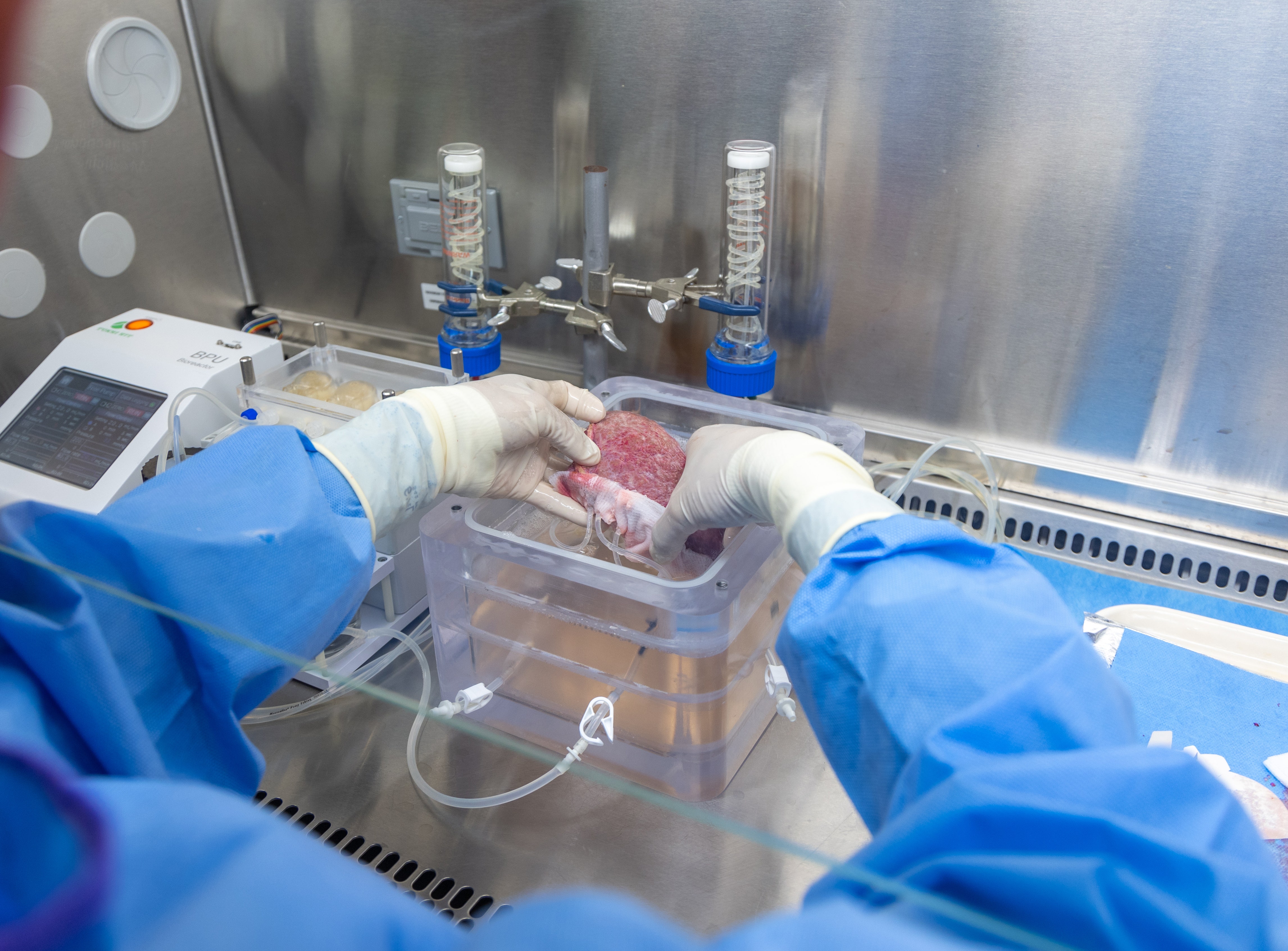
On a recent Thursday afternoon, researchers Lanuza Faccioli and Zhiping Hu wheeled an inconspicuous black and white plastic cooler from an operating room at a hospital in downtown Pittsburgh. Inside was a badly scarred liver, just removed from a 47-year-old ...
But psychedelic compounds are tricky to test in this way because their psychedelic effects are so recognizable to those who take them. In the Lykos trials, around 90 percent of the participants were able to correctly guess whether they received ...