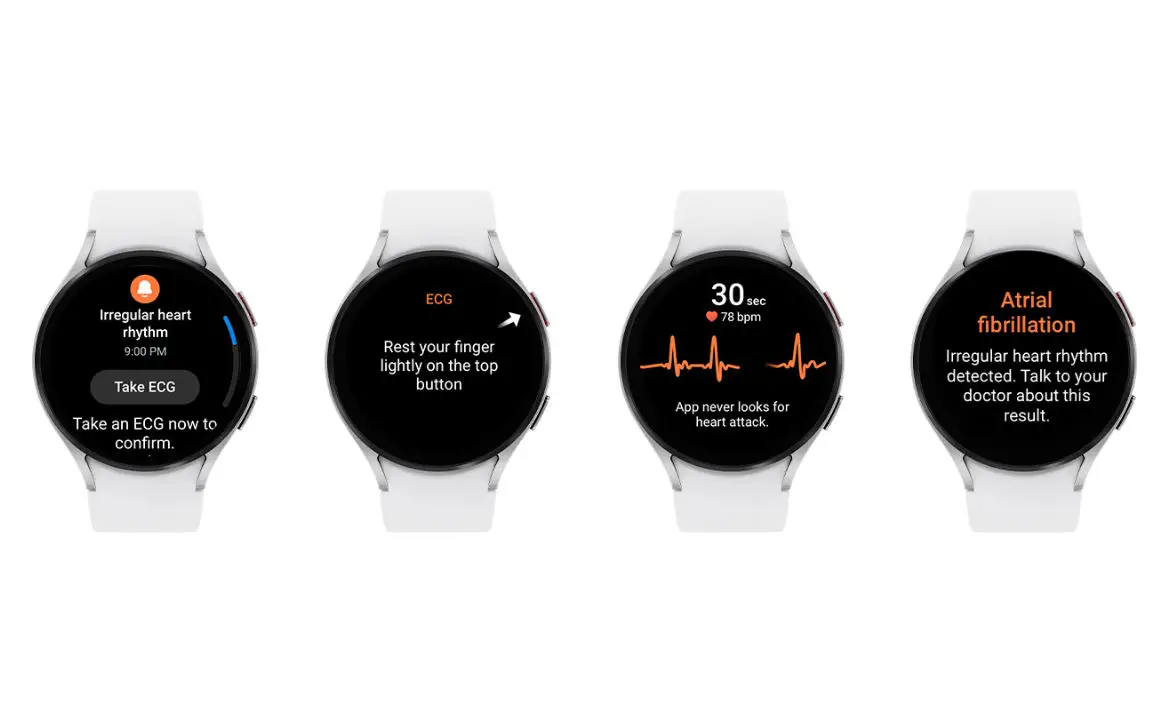
A few days ago, Samsung announced that the FDA had cleared the Galaxy Watch Irregular Heart Rhythm Notification (IHRN) feature. The new IHRN feature works with the Galaxy Watch EKG feature and monitors heart rhythms suggestive of atrial fibrillation (AFib) right from the wrist. Of course, users should be aware that devices such as the Galaxy Watch are not a replacement for a doctor.
Estimated reading time: 2 minutes
According to Samsung, “Cardiovascular disease remains one of the world’s leading causes of death. AFib — a type of arrhythmia — is widely considered a warning sign for major cardiovascular issues that can increase the risk of stroke, heart failure, and other cardiovascular complications.”
The Galaxy Watch offers tools to help users understand their heart health using the Samsung BioActive Sensor. It includes on-demand ECG recording and HR Alert function, which detects abnormally high or low heart rates. But again, this is by no means a replacement for a doctor; always consult your doctor about your health concerns.
Once activated in the Samsung Health Monitor app, the feature will check for irregular heart rhythms in the background via Galaxy Watch’s BioActive Sensor. Suppose a certain number of consecutive measurements are irregular. In that case, Galaxy Watch warns the user of potential AFib activity, prompting them to take an ECG using their watch for a more accurate measurement.
What do you think of this new feature being approved by the FDA? Please share your thoughts on any of the social media pages listed below. You can also comment on our MeWe page by joining the MeWe social network. And subscribe to our RUMBLE channel for more trailers and tech videos.
*We use revenue-generating affiliate links and may earn commissions for purchases made using them. Mentioned pricing is in USD unless otherwise indicated and is accurate at the time of publishing. We often cover brand press releases and those do not constitute an endorsement of any product or service by Techaeris. Only our reviews are an endorsement or lack thereof. Read more on our disclaimer page.
Source link











Leave a Reply